The DEA Has Descheduled Prescription CBD For Severe Epilepsy
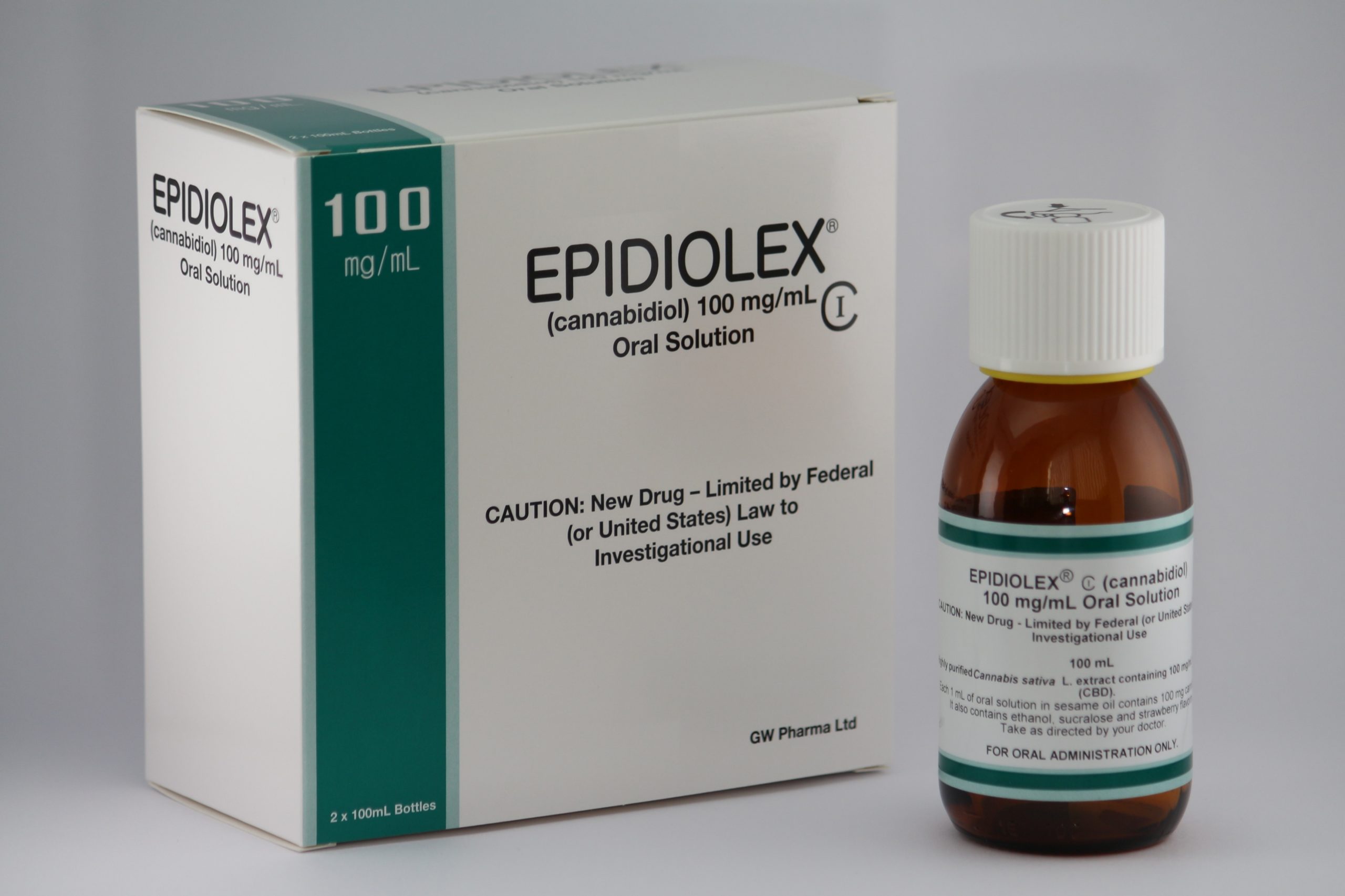
Epidiolex launched in 2018 after earning FDA approval
Parents of children with early-onset epilepsy and their physicians will find it easier to fill their next Epidiolex prescriptions. That’s because the CBD tincture from GW Pharmaceuticals is no longer under the thumb of the Drug Enforcement Agency (DEA).
Non-controlled status
Epidiolex launched in 2018 after earning FDA approval as a treatment to reduce seizure frequency for patients with Lennox-Gastout syndrome and Dravet syndrome — a small population. The 2018 Farm Bill removed hemp-derived CBD from the definition of marijuana in the Controlled Substances Act (CSA), but that didn’t do much to help GW Pharmaceuticals. Epidiolex’s active ingredient is CBD derived from marijuana, not hemp.
The DEA placed Epidiolex in Schedule V, the least restrictive rating a drug can receive under the CSA, but there were still plenty of restrictions. Effective immediately, prescriptions for Epidiolex will be valid for an entire year and can be easily transferred between pharmacies.
To Read The Rest Of This Article On The Motley Fool, Click Here